Contract: THERE 382/2020 (national center for)
PN-III-P2-2.1-PED-2019-3141
The coordinator of the project: – INSTITUTUL NATIONAL DE CERCETARE-DEZVOLTARE IN DOMENIUL PATOLOGIEI SI STIINTELOR BIOMEDICALE „VICTOR BABES”
Partners
P1 – NATIONAL INSTITUTE OF RESEARCH - DEVELOPMENT FOR the NATIONAL IMT-BUCHAREST
P2 – DDS DIAGNOSTICS S. R. L.
The value of the project: 607.500,00 lei
Ongoing : 1.11.2020-31.10.2022
Title
The technology is based on a substrate of nanostructured and functionalized anti-CD36, for the capture of the metastatic tumor cells circulating CTNanoScan
Team
Prof. Dr. Cristiana Tanase,
CSII Dr. Elena Codrici,
CSIII Dr. Popescu Ionela Daniela,
CSIII Drd.Simona Mihai
CSI Dr. Mihaela Gherghiceanu,
CSII Dr. Gheorghita Isvoranu,
CS drd Lucian Albulescu,
Dr.Maria Dudau,
Tehn. Nicoleta Cornelia Constantin,
Tehn. Mihaela Munteanu,
IT Manager Cezar Petrescu,
CSII Dr. Ana-Maria Enciu (project coordinator).
Abstract
The project is based on a concept existing technological (TRL2) – a platform for the capture of stem cells circulating that he will move up to TRL3. Using your knowledge of recent developments in the area of basic research, the project aims to create a substrate of the nanostructured double-functionalized anti-CD36 and anti-EpCAM (Epithelial cell adhesion molecule), in order to increase the catch rate of the metastatic tumor cells in the blood. It brings two improvements to the existing systems are: 1 - to increase the accession of the cell through the nanostructurarea the surface of the 2 - increasing the efficiency of the identification of the cell for it by adding with the functioning of the anti-CD36. The novelty of the project is to increase the sensitivity/specificity of the platform by the functionalization of the substrate for the receptor (CD36), whose expression is consistently present in the cells of the metastatic tumor than just a marker of the classic EpCAM, as used in the concept of the technology available. We have expertise in cell biology, and molecular weight of CS, combined with the expertise of the substrates with nanostructured materials, functionalized, and the fit of their systems, microfluidic to the P1 will result in a product that is going to prove the concept of the project (proof of concept) in the experimental group (TRL3). Our expertise in the validation of new techniques for the diagnosis of cellular IMMului involved in the project will be used in the preparation of the technology for the purposes of validation, next to TRL4.
Summary Of Stages 1-2
They have been selected substrates on the basis of graphite and nanocrystalline, and grafenă drop down, because you don't have related
with the high-affinity cells in the normal and the tumor in the matter, and, at low concentrations, the cell (2000
cells/mL), the rate of binding is less than 5 per cent. This lack of interaction is not specific to
the cell-substrate is the key to the specification provided by the functionality later on.
Certify your best always depended on the type of the structure of the substrate: for the lead
nanocrystalline cysteamine has had the best results, and graphene vertical – protein-A.
the testing of the accession of the cell phones are used for a-line standard-of monocytes in human normal and
line-standardized breast cancer cells. To test the impact of the receptor CD36
the capture of tumor cells, and the efficiency of the substrate, functionalized, what is the purpose of this phase
following this line has been edited genetically engineered to not express this protein.
( read more ...)
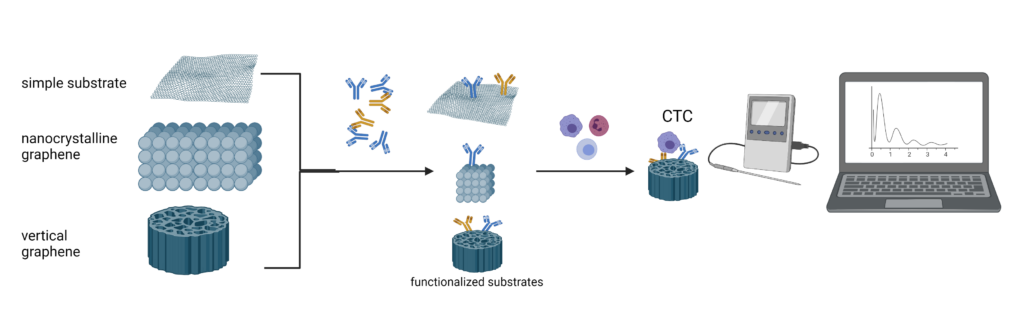
Summary-Stage 3
At this stage, the CO of the tested substrates are functionalized, and nefuncționalizate (given by P1), more than one type of cell, with different profiles of expression of CD36, and EpCAM, in order to see the utility of them in order to capture the various types of cells (MCF-7, and the STAGE 9855), based on the idea that the phenotype of the cell is different between a prison cell for the in situ and cell metastazantă in the blood, or a cell from one site of metastasis. In the end, in order to bring us closer to the biological relevance of our project, we used a sample of human blood from healthy volunteers, we have added a very small number of tumor cells that have been incubated on the substrate are selected in order to test the efficiency of the capture. With the help of partners from the private sector (P2), we came up with a model of a prototype for a rapid determination of the capture of tumor cells in the blood.

