Contract: PED 382/2020 (UEFISCDI)
PN-III-P2-2.1-PED-2019-3141
Coordinator: - NATIONAL INSTITUTE FOR RESEARCH-DEVELOPMENT IN PATOLOGY AND BIOMEDICAL SCIENCES "VICTOR BABES"
Parteners
P1 - NATIONAL INSTITUTE FOR RESEARCH-DEVELOPMENT FOR MICROTECHNOLOGY - IMT
P2 - DDS DIAGNOSTIC S.R.L.
Budget: 607.500, 00 lei
Period:1.11.2020-31.10.2022
Title
Combined CD36 immunoaffinity and nanostructure technology for metastatic tumor cells enrichment from blood CTNanoScan
Team
Prof. Dr. Cristiana Tanase,
CSII Dr. Elena Codrici,
CSIII Dr. Popescu Ionela Daniela,
CSIII Drd.Simona Mihai
CSI Dr. Mihaela Gherghiceanu,
CSII Dr. Gheorghita Isvoranu,
CS drd Lucian Albulescu,
Dr.Maria Dudau,
Tehn. Nicoleta Cornelia Constantin,
Tehn. Mihaela Munteanu,
IT Cezar Petrescu
CSII Dr. Ana-Maria Enciu (coordinator)
ABSTRACT
The project starts from an existing technological concept (TRL2) - platform for capture of circulating stem cells. Using recent basic research knowledge, the project aims to create a nanostructured substrate, functionalized with antibodies against CD36 / EpCAM (Epithelial cell adhesion molecules), to increase the rate of capture of metastatic circulating tumor cells. The project brings two improvements to the already existing systems: 1- increase the cell adhesion by surface nanostructuring and 2- increase the efficiency of metastatic cells identification, by adding the anti-CD36 functionalization to the pre-existing one for EpCAM. The novelty of the project consists in increasing the sensitivity / specificity of the platform by functionalizing the substrate for a receptor (CD36) whose expression is more consistently present on metastatic tumor cells than the classic EpCAM marker, used in the existing technological concept. The expertise in the cellular and molecular biology of CO, combined with the expertise in nanostructured, functionalized substrates and their incorporation into microfluidic systems of P1 will generate a product that will demonstrate the proof of concept at the experimental level (TRL3). The expertise in the validation of some cellular diagnostics techniques of the SME involved in the project will be used to prepare the technology for future validation to TRL4.
SUMMARY REPORT phase 1 and 2
S-au selectat substrate pe bază de grafit nanocristalin și grafenă verticală, deoarece nu au legat
cu afinitate mare celule normale și tumorale în suspensie, iar la concentrații mici de celule (2000
celule/mL), procentul de legare este a fost sub 5%. Această lipsă de interacțiune nespecifică
celulă-substrat este o garanție a specificității oferite de funcționalizarea ulterioară.
Funcționalizarea optimă a depins de tipul de structurare a substratului: pentru grafitul
nanocristalin cisteamina a avut rezultate optime, iar pentru grafena verticală – proteina A. Pentru
testarea aderării celulare au fost folosite o linie standardizată de monocite umane normale și o
linie standardizată de celule de cancer de sân. Pentru testarea impactului receptorului CD36
asupra capturii celulelor tumorale și eficienței substratului funcționalizat, care este scopul etapei
următoare, aceste linii au fost editate genetic să nu mai exprime această proteină.
( read more about it ... )
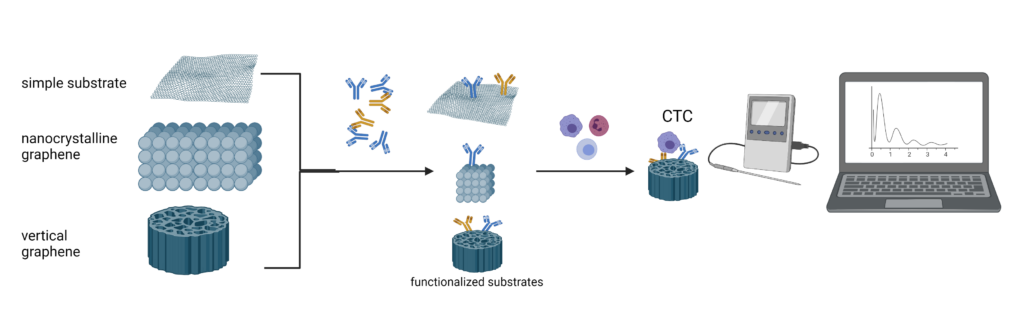
SUMMARY REPORT PHASE 3
At this stage, CO tested functionalized and non-functionalized substrates (manufactured by P1), with several cell types, with different expression profile of CD36 and EpCAM, to see their usefulness for capturing various types of cells (MCF-7 and CRL 9855), starting from the idea that the cell phenotype is different between an in situ cell and a metastatic cell in the blood, or a cell in a metastasis site. Finally, in order to get closer to the biological relevance of the project, we used human blood samples from healthy volunteers, in which we added a very small number of tumor cells, which we incubated on the selected substrates, to test the efficiency of the capture. With the help of private partners (P2), we developed a prototype model for a quick determination of the capture of tumor cells in the blood.

