Protocol for pdt with porphyrins, innovative, and reliable base of the pathology of the skin premalignă - proof pre-clinical
PORPHYDERM
Cod proiect: PN-III-P2-2.1-PED-2021-0360
Contact number: 637PED/2022
Program: Proiect Experimental Demonstrativ (PED)
Domeniu: 1.3 – Biotehnologii
Durata proiectului: 28.06.2022 – 27.06.2024 (24 luni)
Partner institutions
The project is being implemented by a consortium model, which has a long history of collaboration, and expertise in the development of pre-clinical to porphyrins us for photodynamic therapy, as well as in the practice of medicine, and therapy of the base.
- Coordonator: Institutul Național de Cercetare – Dezvoltare în Domeniul Patologiei și Științelor Biomedicale „Victor Babeș”, Director de proiect: CSI Dr. Gina MANDA
- Partener 1: Universitatea de Medicină și Farmacie “Carol Davila”, Responsabil partener P1: Prof. Rica BOSCENCU
- Partener 2: Biotehnos SA, Responsabil partener P2: CSI Dr. Laura OLARIU.
Budget
- The total amount of the budget: 598.795 RON
- The total amount of co-financing (own source): 53.895 RON
- The total value of the Contract: 652.690 RON
Abstract
The project's main objective is the development at the preclinical level, a protocol for the custom of the photodynamic therapy (PDT) using photosensitizers porfirinici innovative and reliable base, make the intelligent, for the treatment of cutaneous precancerous, such as actinic keratosis (AK). The project fits with the theme of Bio – Biotechnology.
The application of medical, will be demonstrated by using cell and mouse models of. It will investigate both the efficacy of the therapeutic and toxicological aspects of the new protocol with DTP. We're gonna get started on the project at the level of TLR 2 is the formulation of the technology/concept to the product, on the basis of a portfolio of six patents, and the expertise of the team in the field of photodynamic therapy and medical base. We will move through a TRL of 3 – proof-of-concept experimental studies, in vitro and ex vivo studies using cell lines and explants from the skin of the mouse. We expect to get to the end of the project, the PARTICIPANTS of 4 – validation of laboratory technology/method, by testing in animal models it has a memorandum of innovative keratoliză by the INS, the application of the AK, which is based on photosensitizers innovative and reliable base.
GOALS
Main objectives
The project's main objective is the development at the preclinical level, a protocol for the custom of the photodynamic therapy (PDT) using photosensitizers porfirinici innovative and reliable base, make the intelligent, for the treatment of cutaneous precancerous, such as actinic keratosis (AK). The project fits with the theme of Bio – Biotechnology.
The application of medical, will be demonstrated by using cell and mouse models of. It will investigate both the efficacy of the therapeutic and toxicological aspects of the new protocol with DTP. We're gonna get started on the project at the level of TLR 2 is the formulation of the technology/concept to the product, on the basis of a portfolio of six patents, and the expertise of the team in the field of photodynamic therapy and medical base. We will move through a TRL of 3 – proof-of-concept experimental studies, in vitro and ex vivo studies using cell lines and explants from the skin of the mouse. We expect to get to the end of the project, the PARTICIPANTS of 4 – validation of laboratory technology/method, by testing in animal models it has a memorandum of innovative keratoliză by the INS, the application of the AK, which is based on photosensitizers innovative and reliable base.
Secondary objectives
The project's main objective is the development at the preclinical level, a protocol for the custom of the photodynamic therapy (PDT) using photosensitizers porfirinici innovative and reliable base, make the intelligent, for the treatment of cutaneous precancerous, such as actinic keratosis (AK). The project fits with the theme of Bio – Biotechnology.
The application of medical, will be demonstrated by using cell and mouse models of. It will investigate both the efficacy of the therapeutic and toxicological aspects of the new protocol with DTP. We're gonna get started on the project at the level of TLR 2 is the formulation of the technology/concept to the product, on the basis of a portfolio of six patents, and the expertise of the team in the field of photodynamic therapy and medical base. We will move through a TRL of 3 – proof-of-concept experimental studies, in vitro and ex vivo studies using cell lines and explants from the skin of the mouse. We expect to get to the end of the project, the PARTICIPANTS of 4 – validation of laboratory technology/method, by testing in animal models it has a memorandum of innovative keratoliză by the INS, the application of the AK, which is based on photosensitizers innovative and reliable base.
THE SCHEME OF THE STUDY
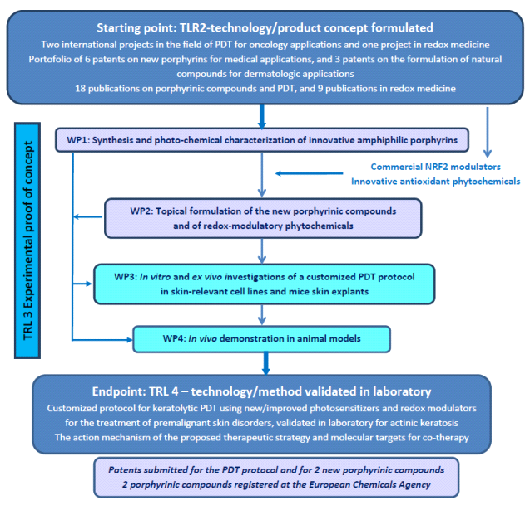
EXPECTED RESULTS
1) At least 2 of the compounds porfirinici us for the treatment of cheratozei keratoses (AK), with improved properties in comparison with the photosensitizers for commercial. Improvements are expected in terms of the amfifilicitatea, fluorescence imaging in vivo to yield a significant singlet oxygen for photodynamic therapy, and the stability is good / the preservation of the properties of pdt in the formulation of the topic of the fotosensibilizatorilor.
2) technical Specifications for all of the compounds of the porfirinici and fitocompușii make, as well as for the INS is selected, and the result of that, in mice, AK.
3) the Documentation for a minimum of 3 patents and patent applications, filed in the Office of the Patent and Trademark office.
4) the Two compounds porfirinici registered with the European Chemicals Agency.
5 a) with At least 2 publications in journals with impact factor > 4, and for at least 2 papers at conferences relevant to the field of dermatology, toxicology, and biology of redox reactions (bear in mind that the results associated with the patent should not be published previously).
6) set up A workshop held at the end of the project is to present the results to stakeholders (academia, r & SMES, pharmaceutical).
7) New research on the pre-clinical testing of new strategies of photodynamic therapy in dermatology, which will be deployed to: IV (PDT on the tumor cells, and small animals out of the lab) and BTH (photodynamic therapy in vitro, the cells that are relevant to the skin, the process for the formulation of the porphyrin, and the modification of the redox photochemical), which is due to be published in the eERIS.
8) the Development of a detailed strategy for the market, leveraging, and by the COST Action CA20121, which establishes the framework for cooperation with SMES, pharmaceutical medicine, redox reactions and the pharmaceutical industry.
9) in the web Page, and the media channels are dedicated to the promotion of the knowledge generated in this project to the professionals, the pharmaceutical industry and the general public.
A SUMMARY OF THE PHASE I/2022
The synthesis and preliminary characterization of the new compounds porfirinici
Statisticile recente evidențiază cancerul ca principală cauză de deces la nivel mondial, prevăzând o creștere a numărului de cazuri față de anul 2020 cu 47% până în anul 2040 [1,2]. Din aceste considerente dezvoltarea de strategii inovative privind diseminarea măsurilor de preventie și furnizarea de substanțe active cu potențial antitumoral sunt esențiale pentru combaterea cancerului la nivel mondial. Leziunile non-melanom (keratoze actinice, carcinom spinocelular și bazocelular, limfom cutanat, sarcom Kaposi, angiosarcom) sunt printre cele mai frecvente forme de cancer. Dezvoltarea unui cancer non-melanom este determinată de factori genetici, istoric de infecție HPV, istoric de afecțiuni cutanate inflamatorii cronice, expunere la substanțe toxice, la soare, imunosupresie, fototipurile cutanate I și II, PUVA ca terapie anterioară [3, 4]. Keratozele actinice sunt descrise in literatura sub forma de ,,carcinom in situ” (din cauza keratinocitelor displazice asemănătoare cu carcinomul cu celule scuamoase) sau ca leziune premalignă [5]. Abordarea terapeutica în cazul keratozei actinice trebuie să ținteasca scăderea riscului de malignizare și un diagnostic precoce al formațiunilor maligne care pot apărea într-un camp de keratoză actinică. Porfirinele, prin profilul lor structural si spectral, au potential în identificarea și terapia manifestărilor cutanate de tip malign [6]. În plus, avantajul major al acestor tipuri structurale asemănătoare hemului este selectivitatea pentru celule tumorale și versatilitatea lor, posibilitatea modelării structurale prin atașare de substituenți cu diferite grade de polaritate, pentru obtinerea unui raport hidrofil/lipofil optim pentru internalizarea celulară.
In acest context, obiectivul principal al proiectului este de a elabora un protocol pentru terapia fotodinamica (PDT) a keratozei actinice, utilizand noi derivati porfiriniici. Activitatile din cadrul proiectului includ evaluarea potentialului de marker si agent antitumoral al unor compusi porfirinici cu arhitectura moleculara asimetrica si profil spectral adecvat. O primă etapă a proiectului prevede obținerea de noi structuri porfirinice, prin tehnici ecologice de sinteză care respectăa cerințele actuale din domeniul sintezei medicamentului. S-a urmărit obținerea unui set de date asociate procedurilor de sinteză și parametrilor care definesc profilul structural al noilor fotosensibilizatori.
In cadrul Activitatii I.1 realizată de UMF au fost obținuti următorii compuși porfirinici:
- with asymmetric structures:
- 5-(2,4-dihidroxifenil)-10,15,20-tris-(4-acetoxi-3-metoxifenil) porfirina (2)
- The 5-(2-hydroxi-3-methoxyphenyl)-10,15,20-tris-(4-carboximetilfenil) porfirina P (5.2)
- cu structuri simetrice (referință pentru derivații asimetrici în studiile in vitro)
- 5,10,15,20-meso-tetrakis-(4-acetoxi-3-metoxifenil) porfirina (P4.1º1)
- 5, 10,15,20-tris-(4-carboximetilfenil) porfirina P (5.1)
The assessment of the structural and the spectrum of the synthesized compounds has been achieved in the Work of I. 1. through the analysis of the NMR, ft-IR, UV-Vis, and fluorescence detection. The correlation of the obtained data confirmed the structures of the compounds porfirinici done in this stage. In conclusion, our preliminary studies have led to the production, good yields of the 4 compounds porfirinici by the approach of a method for the synthesis of the modern and eco-friendly.
În Activitatea I.2 au fost analizate 3 tipuri de formulări pentru aplicații topice ale compușilor porfirinici și modulatorilor, întro primă etapă fiind selecționată și detaliată metoda în gel.
Studiul in vitro realizat în Activitatea I.3 a arătat că toți compușii porfirinici analizați, evaluați in vitro pe celule normale și tumorale specifice pielii (keratinocite umane normale HaCaT, fibroblșsti umani dermici HS27 și celule de mealnom de șoarece B16F10) au avut o bună biocompatibilitate cu celulele normale și tumorale de piele. Se remarcă totuși următoarele aspecte: 1) compusul P5.2 poate inhiba multiplicarea keratinocitelor, ceea ce are relevanță terapeutică în cazul keratozei actinice caracterizată prin hiper-proliferarea acestor celule, dacă se evită fotosensibilizarea temporară a pielii; b) compușii P2.1 și P5.2, dar nu și derivatul porfirinic P4.2, determină creșterea eliberării LDH de către fibroblaștii dermici, posibil datorită unor efecte nedorite care perturbă integritatea membranară a fibroblaștilor dermici.
All of the compounds of the porfirinici review have been incorporated into normal cells, and tumors of the skin were investigated, it is true, to varying degrees, thus proving the properties of the tracer fluorescent. The compounds of the porfirinici P2.1 and P4.2, may be considered as candidates for the imaging of fluorescence in the skin, and for photodynamic therapy in dermatology oncology.
Pentru vizibilitatea proiectului și a realizărilor sale, în Activitatea I.4 a fost realizată pagina web a proiectului, care se găseste la adresa
În Activitatea I.5, coordonată de UMF a fost elaborată cererea de brevet de invenție cu titlul ,,Compus porfirinic cu potential de marcator fluorescent in dermato-oncologie”, la care au participat toți partenerii. Documentatia tehnică a cererii de brevet revendică elemente de noutate corespunzatoare obținerii, evaluării spectrale și biologice a compusului 5-(2-hydroxi-3-metoxifenil)-10,15,20-tris-(4-carboximetilfenil) porfirina (P5.2). Cererea de brevet de invenție a fost înregistrată la OSIM.
The objective of the activities of phase I has been completed 100%.
A SUMMARY OF THE PHASE II/2023
Study of preclinical to intermediate
The activities of phase-II
Activity | Partner |
The activity of the II.1 The synthesis and characterization of compounds porfirinici for pre-clinical studies | PHARMACY (P1) |
The activity of the II.2 Production, formulation and characterization of fitocompusi | BTH (P2) |
The activity of the II.3 Studiu biologic in vitro | IV (SC) |
The activity of the II.4 The wording of the porfirinici and modulators rate for the top | BTH (P2) |
The activity of the II.5 Studiu biologic ex vivo pe explanturi de piele | BTH (P2) |
The activity of the II.6 Development and characterization of an animal model of ankylosing spondylitis | IV (SC) |
The activity of the II.7 Pre-study in vivo studies in the animal model | IV (SC) |
The activity of the II.8 The assessment of the skin, in the car, on the pattern of actinic keratosis treatments | BTH (P2) |
The activity of the II.9 The dissemination of the results to update the web page, article, communication, international co-production. | IV (SC) PHARMACY (P1) |
The results of the phase II
Expected results | Results achieved |
The 2 compounds porfirinici select and that the technical specification of the intermediate | The 3 compounds porfirinici to the specifications of P2.1, P2.2, and P4.2 (see Appendix 1) |
2 fitocompusi to the specifications of the technical | 2 fitocompusi with the technical specifications (Activity II.2 and Appendix 2) |
1 raport final de studiu biologic in vitro | Raport de studiu preliminar in vitro (descris la Activitatea II.3. Rezultatele sunt prezentate in articolul publicat in revista ISI (Anexa 3 si Anexa 9-articol submis pentru publicare) |
1 the report of a study of the intermediate on the explants of the skin | • The method for obtaining and testing explanturilor for your skin, in order to study the diffusion, the vertical axis of the preparations, topical preparations, and transdermal (see Appendix 5.1); • Using the HPLC method for the compounds porfirinici; • The Protocol for the realization of the PDT, the explants of the skin (see Annex 5.2). |
The 2 compounds porfirinici 2 fitocompusi make up for the app top | Variants of the gel formulation of the topic of the fotosensibilizatorilor and fitocompusilor for pacific daylight time, at the level of the skin (the Work of the II.4, Annex 4) |
1 protocol de PDT ex vivo pe explanturi de piele | Protocol de PDT ex vivo pe explanturi de piele (Anexa 5) |
2 a an animal model of ankylosing spondylitis | 2 a an animal model characterized (repeated exposure to UVB radiation, tumors, transplantable cell carcinomas of human skin squamous cell carcinoma in mice, nude imunosupresati); A third model is in the process of development (carcinogenesis, chemically induced in mice FAV). Do you see the Work of, II.6 and in Annex 6. |
1 raport de studiu preliminar in vivo | Raport de studiu preliminar in vivo pe soareci C57BL/6 expusi repetitiv la doze crescatoare de UVB si tratati in vivo cu (Activitatea II.7). |
A Panel of target genes of nrf2 pathway | A Panel of target genes of nrf2 pathway is relevant for photodynamic therapy (Appendix 7.1) |
1 protocol for the combination therapy of PDT and to the modulation rate in the model, preclinical | 1 protocol for the combination therapy of PDT and to the modulation rate in the model of preclinical (see Appendix 7.2) |
1 procedură de investigare a pielii in vivo | The procedure for the investigation of the skin in vivo, and (The, II.8, Annex 8) |
1 the web page is updated on | PORPHYDERM – National Institute of Pathology, Victor Babes university – uttar Pradesh (iv.en) |
2 communications
| · 1 the communication of science to the Scientific Conference for the fall of the ARS 2023, with the title ”Science for a healthy society”, held in the period from 21 to 23.09.2023, "OVIDIUS" University of Constanta; · 1 oral presentation in the scope of the factor of the transcription nrf2 pathway in cancer, the Course of "the nrf2 pathway in the Noncommunicable Diseases: from Bench to Bedside" was organized within the framework of the Action, the COST CA20121 in the period from 26 to 30.06.2023, at smolenice Castle, Slovakia. |
2 scientific papers published | 1 the article was published in the year 2023 in the journal Molecules (ISI, impact factor 2023 4.927); 1 the article is submitted for publication in the journal Pain (ISI, impact factor 5.215). |
2 application for patent filed in the patent OFFICE | 1 the application for the patent was filed at the patent OFFICE, no. for the record, He/00775/28.11.2022 and the title Compound, porphyrin, with the potential of a marker, fluorescent in dermato-oncology” (the deposit be placed in advance of the stage) · For one of the compounds that can be tested in the frame of acetsei stage has been awarded the berevetul No. 132752 B1, and published in the uk-MADE 11 of 29.11.2023, with the title of "Porphyrin derivatives for theranostic use", author: Rica Boscencu, Gina Manda, R. Petre, Socoteanu, Mihail Eugen Hinescu, the Next Victory of the Car, Laura Olariu, Phone Communist. |
1 application for patent in the course of development | 1 application for a patent in the course of the preparation for making fotosensibilizatorilor selected in steps 1 and 2 of the project, to the application is off-topic in pacific daylight time. |
The 2 protocols in collaboration with experts from abroad. | 3 the protocols of cooperation with the experts from abroad. |
A SUMMARY OF THE PHASE III/2024
The study of the validation of the obtained results
The activities of this phase III
Activity | Partner |
The work of the third.1 The synthesis and characterization of the components of the porfirinici will be responsible for the validation study |
PHARMACY (P1) |
The work of the third.2 Production, formulation, and characterization of the compound of the porphyrin and the fitocompusului selected for the validation study |
BTH (P2) PHARMACY (P1) |
The work of the third.3 Studiu biologic de validare ex vivo a terapiei combinate PDT si modulare redox pe explanturi de piele de la soareci cu cheratoza actinica |
BTH (P2) IV (SC) |
The work of the third.4 Studiu biologic de validare in vivo a terapiei combinate PDT si modulare redox la soareci cu cheratoza actinica |
IV (SC) |
The work of the third.5 The dissemination of the results of the | IV (SC) PHARMACY (P1) |
The work of the third.5.1 To update a web page, creating a protocol for the transfer of technology between the partners, and the organization of a workshop, demonstration |
IV (SC) |
The work of the third.5.2 Drawing up the technical documentation to the final of the preclinical study and the dissemination of its |
PHARMACY (P1) |
The results of the phase III
Expected results | Results achieved |
1 compound, porphyrin validated and the specification of its technical final | Compusul porfirinic P2.2 validat in vitro si in model animal de carcinomatoza chimic indusa Compusii porfirinici P2.1 si P4.2 validati in vitro The technical specifications of this product on P2.1, P2.2, and P4.2 |
1 compound, porphyrin is formulated | The 2 compounds porfirinici ("P2".1, and P2.2) is formulated in a gel for topical (drugs and the hidroxipropilmethilceluloza) 2 the documentation for a patent for the gels with porfirina |
1 fitocompus made the specification to the technical | 1 fitocompus is formulated and the data sheet for the product |
1 report of the validation study ex vivo | 1 raport de studiu in vivo in model animal de soareci expusi cronic la UVB The basis of the data with the parameters of the skin are determined by non-invasive methods in mice exposed to chronic UVB rays, and are treated with photodynamic therapy or modification of the redox |
1 protocol for the investigation ex vivo the skin of the | Protocol de investigare ex vivo a pielii |
1 the study of the biological validation in vivo studies | Validarea in vivo a compusului porfirinic P2.2 in model animal de cheratoza actinica (carcinomatoza indusa chimic) si in model animal de expunere cronica la UVB |
1 is a portfolio of experimental methods | The components of the porfirinici · Physico-chemical characterization of the components of the porfirinici · The physical, chemical, and pharmaceutical gels with porphyrins for the top · PDT in-vitro and in vivo studies · The procedure for the investigation, a non-invasive skin of the rat |
1 page date | PORPHYDERM – National Institute of Pathology, Victor Babes university – uttar Pradesh (iv.en) |
1 protocol for the transfer of technology between partners | Protocol de transfer de cunoastere dinspre IVB (CO) catre BTH (P2) privind testarea biologica in vitro a fotosensibilizatorilor pentru terapie fotodinamica. |
1 application for patent filed in the patent OFFICE | 2 application for patent filed in the patent OFFICE · The application for the patent A00567 of 23.09.2024 Porphyrin asymmetry in the matrices of hydroxypropylmethylcellulose for the treatment of cutaneous precancerous. Authors: Emma Adriana's Data Andrea Michaela Burloiu, Rica Boscencu, Gina Manda, V Announced, Cristina-Elena Dinu, Pirvu, Dumitru Lupuliasa, T, Is The Next Victory, Adina Magdalena Musuc, Mihai Anastasescu, R. Petre, Socoteanu. · The application for the patent A00630 of 23.10.2024 of the Hydrogel with the porfirina in combination with chitosan for potential applications in the dermatology and oncology. Authors: Pleased To Laura, Boscencu Rica, Manga, Gina, S Is Burloiu, Andreea-Mihaela Mihai Dragos-Paul, Sandy To Her, Diana. A patent obtained Derivative of porphyrin to the use of the teranostică. Authors: Rica Boscencu, Gina Manda, Radu Socoteanu, Mihail Eugen Hinescu, The Next Victory Of The Car, Laura Olariu, Phone Communist. RO132752 (A0) 2018-08-30, RO132752 (1) 2023-11-29. |
The 2 compounds porfirinici sign in to the European Agency of the Chemicals | It is, in the course of preparing the documentation for the registration of the 2 compounds porfirinici ("P2".1, and P2.2), but it has made a toxicological study of the broader uncertainty in the project. The ship, which has not been completed on the project, it is cleared by the 2-publications and ITS supplements. |
1 workshop for a demonstration | Workshop, exhibition, organized the IV (CO -) at the time of the 8.10.2024: The PORPHYDERM the short-term (Gina Manda, IV) · Modelare in silico pentru compusi porfirinici (Dragos Mihai, UMF) · The compounds porfirinici innovation for the photodynamic therapy of diseases of the skin, non-malign (Rica Boscencu, and PHARMACY) · Patent protection on the compound of the P4.2 – the gold Medal in the E-t-t-A-I-N-V E N T-2024 (Laura was Pleased BTH) · The compounds porfirinici incorporated into the gel for the topical (Sabina S. k., BTH) · Discussions and plans for the future |
The results of the additional | 3 articles: 1. Assessment of some unsymmetrical porphyrins as promising molecules for photodynamic therapy of cutaneous disorders. Burloiu, A. M., Ma, G., Lupuliasa, D., Socoteanu, P. R., Michael, D. P. T, S. V. D., L. I. A, Surcel, M. Anastasescu, M., Olariu, L., Gîrd, C. E., Barbuceanu, S. F., Ferreira, L. F. V., Boscencu, Republic Of Pain, 17(1), 62, 2024 (I. F.=4.3, Q1). This research was supported by the Romanian Ministry of Research, Innovation and Digitalization through the grant 637PED/2022, and the Nucleus of the grant PN-23.16.02.01/2023. 2. In-silico and in-vitro studies, the asymmetrical porphyrin derivatives with therapeutic potential in skin disorders. Burloiu, A. M., Michael, D. P., Ma, G., Lupuliasa, D, T,,. I. V., Socoteanu, R. P., Surcel, M. D., L. I., Olaru, L., Gîrd, C. E., Boscencu, Republic Of Pain, 17(6), 688, 2024 (I. F.=4.3, Q1). This research was supported by the Romanian Ministry of Research, Innovation and Digitalization through the grant 637PED/2022, the Nucleus and the grant of the PN 23.16.02.01/2023", " and from the “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania. 3. Porphyrin photosensitizers into polysaccharide-based biopolymer hydrogels for topical Photodynamic Therapy: Physicochemical and pharmacotechnical assessments. Burloiu, A. M., Ozone, E. A., Musuc, A. M. Anastasescu, M., Socoteanu, R. P., Atkinson, J., Culita, D., Tell, V., Smith, J. A., Lupuliasa, D., Michael, D. P., Gîrd, C. E., Boscencu, R., Gels, 2024, 10, 499. (I. F.=5, Q1,). This research was supported by the Romanian Ministry of Research, Innovation, and Digitization. |
2 the works of releases: · The 16th European Exhibition of Creativity and Innovation, Iasi, Romania, from 6 to 8 June 2024: Porphyrynic Derivatives for the Theranostics of Use Patent No. 132752 B1 and friends in the-GAZETTE, may 11, from 29 to November 2023, Laura Was Pleased Rica Boscencu, Gina Manda, Radu Socoteanu, Mihail Eugen Hinescu, The Next Victory Of The String, Phone: Communist. Communication is the poster, which has received the A gold medal. · 23rd Romanian International Conference on Chemistry and Chemical Engineering (RICCCE), Mamaia, Constanta, Romania, On 4-7 September, 2024: Morphological and spectral assessment of an unsymmetrical porphyrinic complex with biomedical potentialElena Christen Branch Rica Boscencu, Roxana Trusca, Adina Magdalena Musuc, Radu Socoteanu, Mihai Anastasescu. |
The dissemination of the project PORPHYDERM
• Website: PORPHYDERM – National Institute of Pathology Victor Babeş – Bucharest (ivb.ro)
• 3 cereri de brevet depuse la OSIM:
- 3 patents and patent applications filed in the patent OFFICE:
- Compound, porphyrin, with the potential of the fluorescent marker in dermato-oncology. Authors: Burloiu, Andreea Mihaela, Ma Gina, Boscencu Rica-T, The Next Victory, The Lupuliasa Demetrius, Surcel Michael Was Pleased, Laura, Michael Dear Paul. Patent application no. 202200775, to be published in the uk-MADE 5/30.05.2023.
- Porphyrin asymmetry in the matrices of hydroxypropylmethylcellulose for the treatment of cutaneous precancerous. Authors: Emma Adriana's Data Andrea Michaela Burloiu, Rica Boscencu, Gina Manda, V Announced, Cristina-Elena Dinu, Pirvu, Dumitru Lupuliasa, T, Is The Next Victory, Adina Magdalena Musuc, Mihai Anastasescu, R. Petre, Socoteanu. Patent application A00567 of 23.09.2024.
- The hydrogel with the porfirina in combination with chitosan for potential applications in the dermatology and oncology. Authors: Pleased To Laura, Boscencu Rica, Manga, Gina, S Is Burloiu, Andreea-Mihaela Mihai Dragos-Paul, Sandy To Her, Diana. Patent application A00630 of 23.10.2024.
- 4 of the articles published in ISI journals, and 3 in Q1, and 1 in Q2:
- Porphyrin macrocycles: general, properties, and theranostic potential. Boscencu, R., Radulea, N., Ma, G., Machado, I. F., Socoteanu, R. P., Lupuliasa, D., Burloiu, A. M., Michael, D. P., Ferreira, L. F. V., Molecules, 28(3), 11492023, 2023 (I. F.=4.2, Q2). The research was supported by the Ministry of Research, Innovation and Digitalization, Romania, and through the PORPHYDERM project (sta. no. 637PED/2022), and the Nucleus of the project PN-23.16.02.01/2022.
- Assessment of some unsymmetrical porphyrins as promising molecules for photodynamic therapy of cutaneous disorders. Burloiu, A. M., Ma, G., Lupuliasa, D., Socoteanu, P. R., Michael, D. P. T, S. V. D., L. I. A, Surcel, M. Anastasescu, M., Olariu, L., Gîrd, C. E., Barbuceanu, S. F., Ferreira, L. F. V., Boscencu, Republic Of Pain, 17(1), 62, 2024 (I. F.=4.3, Q1). This research was supported by the Romanian Ministry of Research, Innovation and Digitalization through the grant 637PED/2022, and the Nucleus of the grant PN-23.16.02.01/2023.
- In silico - and - in-vitro study of the asymmetrical porphyrin derivatives with therapeutic potential in skin disorders. Burloiu, A. M., Michael, D. P., Ma, G., Lupuliasa, D, T,,. I. V., Socoteanu, R. P., Surcel, M. D., L. I., Olaru, L., Gîrd, C. E., Boscencu, Republic Of Pain, 17(6), 688, 2024 (I. F.=4.3, Q1). This research was supported by the Romanian Ministry of Research, Innovation and Digitalization through the grant 637PED/2022, the Nucleus and the grant of the PN 23.16.02.01/2023", " and from the “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
- Porphyrin photosensitizers into polysaccharide-based biopolymer hydrogels for topical Photodynamic Therapy: Physicochemical and pharmacotechnical assessments. Burloiu, A. M., Ozone, E. A., Musuc, A. M. Anastasescu, M., Socoteanu, R. P., Atkinson, J., Culita, D., Tell, V., Smith, J. A., Lupuliasa, D., Michael, D. P., Gîrd, C. E., Boscencu, R., Gels, 2024, 10, 499. (I. F.=5, Q1). This research was supported by the Romanian Ministry of Research, Innovation, and Digitization.
- Communications
Oral presentations
1 the communication of science to the Scientific Conference for the fall of the ARS 2023, with the title ”Science for a healthy society”, held in the period from 21 to 23.09.2023, "OVIDIUS" University of Constanta;
In the framework of the Action, the COST CA20121 "the nrf2 pathway in the Noncommunicable Diseases: from Bench to Bedside", from 26 to 30 June 2023, Smolenice Castle, Slovakia. The nrf2 pathway in cancer, and radiotherapyGina Manga.
Poster
- 16th European Exhibition of Creativity and Innovation, Iasi, Romania, from 6 to 8 June, 2024. Porphyrynic Derivatives for the Theranostics of Use Patent No. 132752 B1 and friends in the-GAZETTE, may 11, from 29 to November 2023, Laura Was Pleased Rica Boscencu, Gina Manda, Radu Socoteanu, Mihail Eugen Hinescu, The Next Victory Of The String, Phone: Communist. Gold medal
- 23rdRomanian International Conference on Chemistry and Chemical Engineering (RICCCE), Mamaia – ConstantaIn Romania, from 4 to 7 September, 2024. Morphological and spectral assessment of an unsymmetrical porphyrinic complex with biomedical potentialElena Christen Branch Rica Boscencu, Roxana Trusca, Adina Magdalena Musuc, Radu Socoteanu, Mihai Anastasescu.
- 3 letters of collaboration
A letter of cooperation from the Teacher. Antonio Cuadrado, Faculty of Medicine, University Autonoma of Madrid & Institutes of Biomedical Research, the Ciudad Universitaria de Cantoblanco, 28049 Madrid, Spain & C. Arturo Duperier, 4, 28029 Madrid, Spain e-mail: antonio.cuadrado@uam.es. The scope of the collaboration, the activation of the transcription factors nrf2 pathway in photodynamic therapy, and possibilities of the modulating factors of the transcription factor.
A letter of collaboration from Devrim Pesen Okvur, Izmir Institute of Technology, Gülbahçe, Izmir Yüksek Teknoloji Enstitüsü, 35433 Urla/Izmir, Turkey, e-mail: devrimpesen@iyte.edu.tr. The scope of co-operation, development of advanced methods for the type of lab-on-a-chip, in order to investigate the in-vitro the therapy of pdt.
A letter of cooperation from the Teacher. Prof. Luís Filipe Vieira Ferreira, Universidade de Lisboa, Centro de Química-Física Molecular, Complexo Interdisciplinar, Instituto Superior Técnico, Av. Rovisco Pais 1049-001, Lisboa, Portugal E-Mail: LuisFilipeVF@ist.utl.pt Tel: 351-21 84 19 252 / 246 / 039. The field of co-creation: the design and characterization of the new compounds porfirinici for photodynamic therapy.
- The participation of the countries of the european
The action of the CHARGE CA20121 "Bench-to-Bedside transition for the Pharmacological regulation of the nrf2 pathway in the noncommunicable diseases", which is an acronym BenBedPhar, 2021-2025, Including: Univ. Antonio Cuadrado, Autonomous University of Madrid, Spain, and the Vice-chair: Gina Ma (director of the PED), Grant Holder: PATHOLOGY, ”Victor Babeş”, Usa (CO-project of the PED).
- 2 ph. d. thesis in the area of the completed project
- Nanosystems metal-functionalized for the teranostică cancer, 2023. Ph. d. student Help-Iliuta D., ph. d. supervisor, Prof. Univ. Dr Maria crivineanu affiliation: (USAMVB);
- Interdisciplinary studies on these compounds tetrapirolici with the potential for application in dermatology oncology, 2024. Author: Andreea Mihaela Burloiu, ph. d. supervisor: Prof. Dr Dumitru Lupuliasa ("Carol Davila"university).
- Ph. d. students in the area of the project, THERE
A phd student of the farm. S Sabina ("P2"): the Research on the synthesis and characterization of hydrogels for applications in the pathology of epithelial tissue - scientific coordinator: Prof. Dr. Rica Boscencu (P1).
